
4 We speculate that, following the vaccine administration, lipid nanoparticles containing the vaccine mRNA are carried to mammary glands via hematogenous and/or lymphatic routes. In rats, up to 3 days following intramuscular administration, low vaccine mRNA levels were detected in the heart, lung, testis, and brain tissues, indicating tissue biodistribution. Little has been reported on lipid nanoparticle biodistribution and localization in human tissues after COVID-19 mRNA vaccination. These data demonstrate for the first time to our knowledge the biodistribution of COVID-19 vaccine mRNA to mammary cells and the potential ability of tissue EVs to package the vaccine mRNA that can be transported to distant cells. The sporadic presence and trace quantities of COVID-19 vaccine mRNA detected in EBM suggest that breastfeeding after COVID-19 mRNA vaccination is safe, particularly beyond 48 hours after vaccination.
Scientific Discovery and the Future of Medicine.Health Care Economics, Insurance, Payment.Clinical Implications of Basic Neuroscience.Challenges in Clinical Electrocardiography.COVID-19 vaccines for kids: What you need to know.COVID-19 vaccine: Should I reschedule my mammogram?.COVID-19 drugs: Are there any that work?.Centers for Disease Control and Prevention. Novavax COVID-19, adjuvanted vaccine: Overview and safety.CDC recommends use of Johnson & Johnson's Janssen COVID-19 vaccine resume.Recommendation to pause use of Johnson & Johnson's Janssen COVID-19 vaccine.Janssen COVID-19 vaccine: Fact sheet for healthcare providers administering vaccine.National Institute of Allergy and Infectious Diseases. Understanding viral vector COVID-19 vaccines.Understanding how COVID-19 vaccines work.The different types of COVID-19 vaccines.The FDA has also given emergency use authorization to the Novavax COVID-19, adjuvanted vaccine for people age 12 and older. The FDA has given emergency use authorization to the Janssen/Johnson & Johnson COVID-19 vaccine for certain people age 18 and older. The FDA has given emergency use authorization to Moderna COVID-19 vaccines for age 6 months to age 17. The FDA has also approved the Moderna vaccine, now called Spikevax, to prevent COVID-19 in people age 18 and older. The vaccine is under an emergency use authorization for children age 6 months through age 11. Food and Drug Administration (FDA) has approved the Pfizer-BioNTech COVID-19 vaccine, now called Comirnaty, to prevent COVID-19 in people age 12 and older. The Novavax COVID-19 vaccine is a protein subunit vaccine. If you later become infected with the COVID-19 virus, the antibodies will fight the virus.
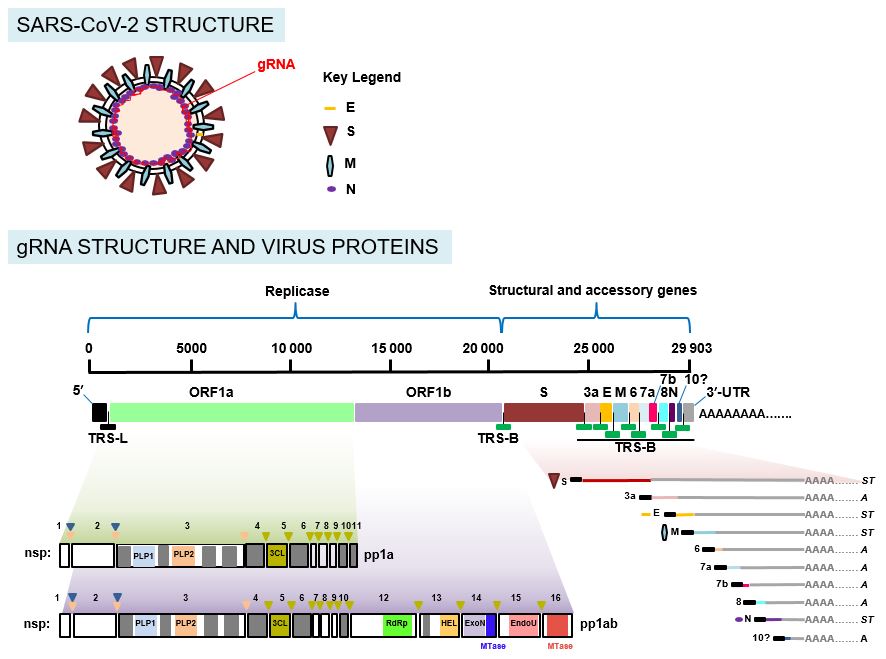
Once your immune system recognizes the S proteins, it creates antibodies and defensive white blood cells. This type of COVID-19 vaccine contains harmless S proteins. Subunit vaccines include only the parts of a virus that best stimulate your immune system. AstraZeneca and the University of Oxford also have a vector COVID-19 vaccine.
The Janssen/Johnson & Johnson COVID-19 vaccine is a vector vaccine. Viral vector vaccines can't cause you to become infected with the COVID-19 virus or the viral vector virus. Once your cells display the S proteins on their surfaces, your immune system responds by creating antibodies and defensive white blood cells. The viral vector gives your cells instructions to make copies of the COVID-19 S protein. In this type of vaccine, material from the COVID-19 virus is placed in a modified version of a different virus (viral vector). Both the Pfizer-BioNTech and the Moderna COVID-19 vaccines use mRNA. The mRNA in the vaccine doesn’t enter the nucleus of the cell, where DNA is kept. Once the protein pieces are made, the cells break down the instructions and get rid of them.


 0 kommentar(er)
0 kommentar(er)
