

We can rotate a VSEPR structure in 3D space and it will still be correct, as long as we do not change the angle between the bonds. Finally, in the practice question above you should realize that there is more than one possible orientation for a VSEPR structure. Even if the three lone pairs are drawn with angles of 90° it is implied that the reader will assume 109.5° just like in the case of the water molecule above.ģ.
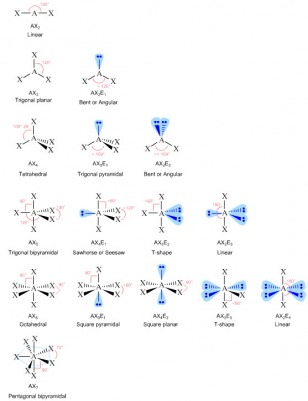
In the case of Cl, like above, the three lone pairs are just drawn around the atom, without trying to show an angle of 109.5° since this is hard to show without being able to draw a wedge or hash. ALL lone pairs should always be shown even on terminal atoms within both a Lewis and VSEPR structure. the VSEPR geometry indicates the correct bond angles (120°), unlike the Lewis structure shown above.Ģ. There are three points that this above practice question is making:ġ. Because no bond angle can be seen around the oxygens even though they have an electron-pair geometry they DO NOT have a molecular shape. We would NOT refer to this as a bond angle as with only one bonding domain the oxygen does not have a bond angle around it. Using VSEPR theory, to put these three domains as far away from each other as possible we would have a trigonal planar electron-pair geometry and angles of approximately 120° between each domain. This means each oxygen has three electron domains (3 regions of electron density). Both oxygen atoms have a double bond to carbon and two sets of lone pairs. Both of these are the same since there are no lone pairs on the C atom.Īround the oxygen atoms, we have a different story though since we have lone pairs we the electron-pair geometry is not the same as the molecular shape. The C in CO 2 has a linear electron-pair geometry and a linear molecular structure/shape. Using VSEPR theory, we predict that the two regions of electron density arrange themselves on opposite sides of the central atom with a bond angle of 180°. (a) We can write the Lewis structure of CO 2 as any of the five Lewis structures shown or variations on these since bond angles do not matter in a 2D Lewis structure, however angles and locations do matter in the 3D VSEPR structure and only one of the five drawings is correct for the VSEPR structure of this molecule:Īround the carbon atom, we have two regions of high electron density, as each double bond counts as one bonding domain (region). (b) boron trichloride, BCl 3, an important industrial chemical (a) carbon dioxide, CO 2, a molecule produced by the combustion of fossil fuels
Predict the electron-pair geometry and molecular structure for each of the following: Predicting Electron-pair Geometry and Molecular Structure: CO 2 and BCl 3 In this case, the molecular structure is identical to the electron pair geometry. The following examples illustrate the use of VSEPR theory to predict the molecular structure of molecules or ions that have no lone pairs of electrons. In an octahedral arrangement with two lone pairs, repulsion is minimized when the lone pairs are on opposite sides of the central atom. In trigonal bipyramidal arrangements, repulsion is minimized when every lone pair is in an equatorial position. If more than one arrangement of lone pairs and chemical bonds is possible, choose the one that will minimize repulsions, remembering that lone pairs occupy more space than multiple bonds, which occupy more space than single bonds. Use the number of lone pairs to determine the molecular structure/shape ( ).Identify the electron-pair geometry based on the number of regions of electron density: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral (, first column).Remember, a single, double, or triple bond counts as one region of electron density since they are connecting the central atom to a single terminal atom. Count the number of regions of electron density (lone pairs and bonds) around the central atom.Write the Lewis structure of the molecule or polyatomic ion.The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures:


 0 kommentar(er)
0 kommentar(er)
